An Asian Hub for the Development and Manufacturing of Cell & Gene Therapies
About Xellera

Rapid Expansion of Cell and Gene Therapy (CGT) Market
– CGT is a revolution of therapeutic innovations in the healthcare and life sciences industry
– Unlike conventional pharmacological (drug) therapies, which are often required throughout the duration of the disease, CGT is designed to be one-off
– Gene therapy involves the delivery of a vehicle to engineer the patient’s gene so as to treat or stop a disease
– Cell therapy involves the transplantation of human cells to replace or repair non-regenerative damaged tissues
State-of-the-art cGMP facilities for Cell and Gene Therapy Products
Hong Kong’s first-of-its-kind cGMP facilities
Compliant with USFDA, EMA, and PIC/S
How are cGMP and GMP different?
At the most basic level, GMP stands for Good Manufacturing Practice, standards set by regulatory agencies for ensuring the quality and safety of the manufactured drug products; the “c” of cGMP stands for current, designating the most advanced version of GMP with the latest technologies, regulations and rules.
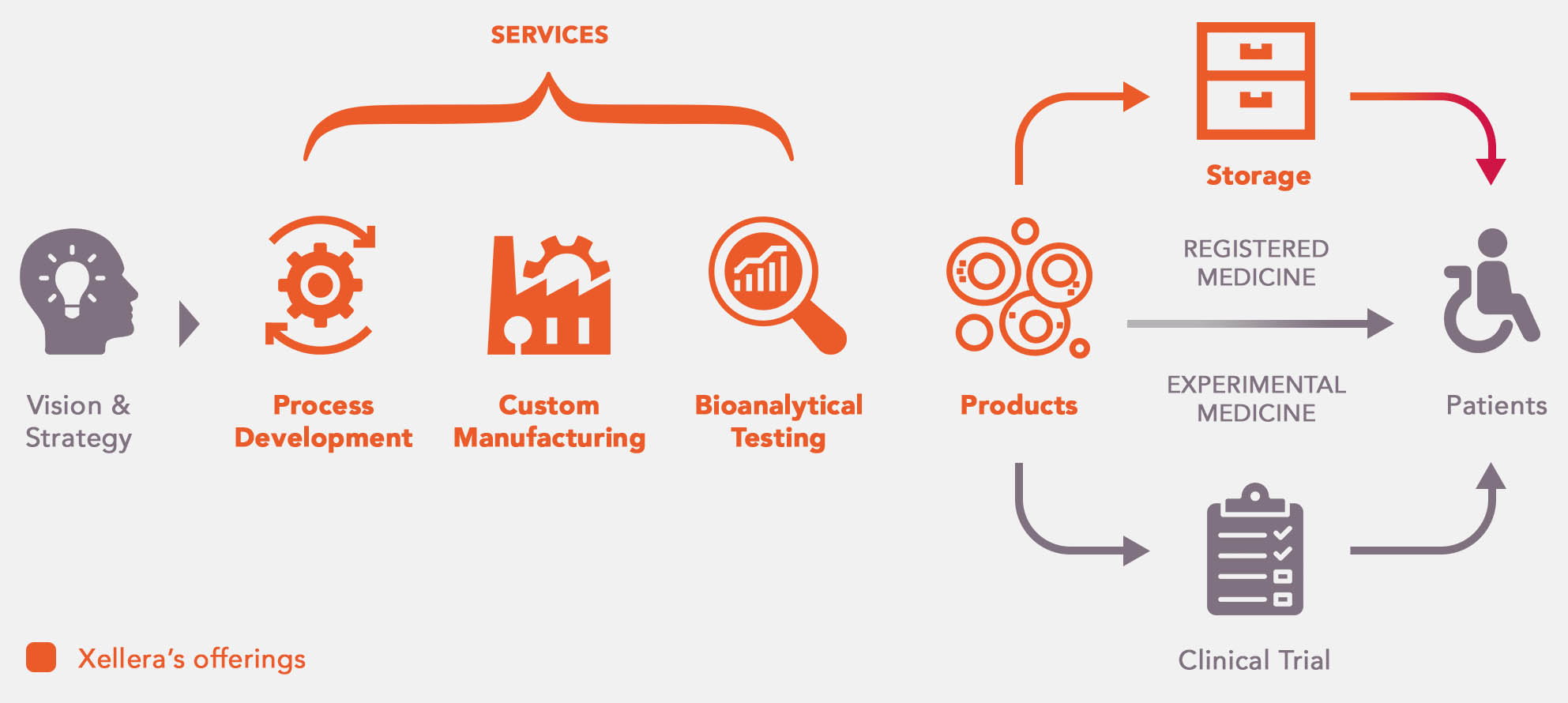
Our CDMO Platform
At Xellera, we understand that the clinical and commercial success of cell and gene therapy products heavily hinges on the successful development of robust, reproducible, and scalable manufacturing processes, in addition to the years or even decades of scientific research. With the extensive experiences and know-how, Xellera aspires to provide our partners an one-stop solution to cGMP manufacturing, spanning from early process development to manufacturing and bioanalytical testing, product release as well as cryostorage.
As a one-stop Contract Development & Manufacturing Organization (CDMO) with our state-of-the-art technology platforms, Xellera accelerates the development and manufacturing of innovative cell and gene products for patients.
Xellera Manufacturing Facility
Unit 810-813, 8/F.,
15 Science Park West Avenue,
Hong Kong Science Park, Shatin,
New Territories, Hong Kong
Xellera Quality Control Lab
Unit 1801, 09-16, 18/F.,
Millennium City 5,
418 Kwun Tong Road,
Kwun Tong, Kowloon, Hong Kong

